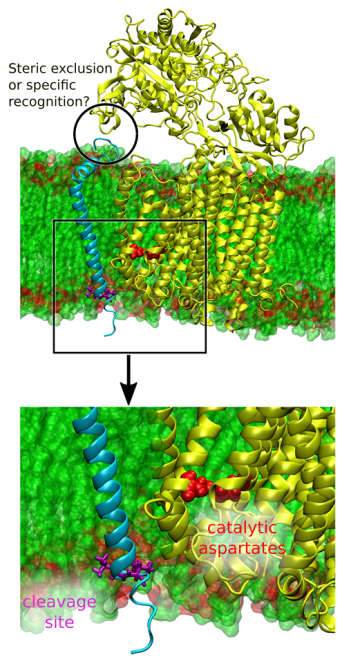
γ-secretase is a membrane-bound enzyme that participates in multiple signaling pathways in the cell by cleaving multiple other proteins. Aberrant processing of one of them, the amyloid precursor protein, by γ-secretase leads to an increased release of β-amyloid polypeptides, which accumulate to form neuritic plaques that are the pathological hallmark of Alzheimer's disease.
The detailed mechanism of action of γ-secretase has remained unrecognized mainly due to a lack of the molecular structure of the enzyme. The latter limitation has recently been mitigated thanks to a rapid development of electron microscopy methods, which allowed for the molecular structure of the enzyme to be resolved in 2015. This structural insight, combined with biochemical results, allowed a range of hypotheses to be proposed about the mechanism of the enzyme activity. Since the experimental verification of these proposals is very difficult, in this research project we intend to use computer simulations to examine how γ-secretase binds its substrate protein before the actual cleavage (i.e., a chemical reaction) takes place. This initial stage of the substrate processing is extremely important as it determines which membrane proteins can be cleaved by γ-secretase.
The aim of the project is to quantitatively characterize the mechanism of substrate recognition and binding by γ-secretase. Finding the structure of the enzyme-substrate complex (a transient state before the cleaving reaction occurs) will allow us to explore the crucial interactions that govern the binding process. In the future, these findings are expected to facilitate the development of new anti-Alzheimer drugs that interfere with substrate binding and therefore lower the level of β-amyloid polypeptides in brain, preventing the formation of toxic aggregates. Furthermore, we intend to investigate the substrate recognition process, which may also be a target for a novel γ-secretase modulators. These modulators could, in principle, show a specificity against the amyloid precursor protein, preventing the occurrence of the side effects associated with inhibition of γ-secretase activity. The obtained results will also extend our understanding of the principles underlying the activity of membrane-bound enzymes.